21
дек
Stoichiometry Program
Posted:adminStoichiometry Course Chapters • (REQUIRED FOR CREDIT!) • • • • • • Section Tests Useful Materials Online Calculators Related Information Links Stoichiometry Stoichiometry is simply the math behind chemistry. Given enough information, one can use stoichiometry to calculate masses, moles, and percents within a chemical equation. • • • • • • • • In chemistry, we use symbols to represent the various chemicals. Success in chemistry depends upon developing a strong familiarity with these basic symbols.
Stoichiometry measures these quantitative relationships, and is used to determine the amount of products and reactants that are produced or needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction.
For example, the symbol 'C'represents an atom of carbon, and 'H' represents an atom of hydrogen. To represent a molecule of table salt, sodium chloride, we would use the notation 'NaCl', where 'Na' represents sodium and 'Cl' represents chlorine. We call chlorine 'chloride' in this case because of its connection to sodium. You should have reviewed naming schemes, or nomenclature, in earlier readings. A chemical equation is an expression of a chemical process. For example: AgNO 3(aq) + NaCl(aq) ---> AgCl(s) + NaNO 3(aq) In this equation, AgNO 3 is mixed with NaCl.
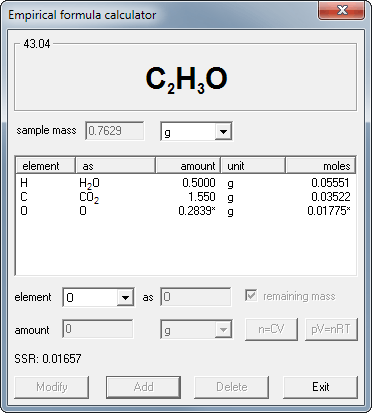
The equation shows that the (AgNO 3 and NaCl) react through some process (--->Download game sepak bola winning eleven 2013. ) to form the (AgCl and NaNO 3). Since they undergo a chemical process, they are changed fundamentally. Often are written showing the that each substance is in. The (s) sign means that the compound is a solid. The (l) sign means the substance is a liquid.
The (aq) sign stands for aqueous in water and means the is dissolved in water. Finally, the (g) sign means that the compound is a gas. Coefficients are used in all chemical equations to show the relative amounts of each substance present. This amount can represent either the relative number of, or the relative number of moles (described below). If no coefficient is shown, a one (1) is assumed. On some occasions, a variety of information will be written above or below the arrows. This information, such as a value for temperature, shows what conditions need to be present for a reaction to occur.
For example, in the graphic below, the notation above and below the arrows shows that we need a chemical Fe 2O 3, a temperature of 1000° C, and a pressure of 500 atmospheres for this reaction to occur. The graphic below works to capture most of the concepts described above.
Popular Posts
Stoichiometry Course Chapters • (REQUIRED FOR CREDIT!) • • • • • • Section Tests Useful Materials Online Calculators Related Information Links Stoichiometry Stoichiometry is simply the math behind chemistry. Given enough information, one can use stoichiometry to calculate masses, moles, and percents within a chemical equation. • • • • • • • • In chemistry, we use symbols to represent the various chemicals. Success in chemistry depends upon developing a strong familiarity with these basic symbols.
Stoichiometry measures these quantitative relationships, and is used to determine the amount of products and reactants that are produced or needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction.
For example, the symbol \'C'represents an atom of carbon, and \'H' represents an atom of hydrogen. To represent a molecule of table salt, sodium chloride, we would use the notation \'NaCl\', where \'Na\' represents sodium and \'Cl\' represents chlorine. We call chlorine \'chloride\' in this case because of its connection to sodium. You should have reviewed naming schemes, or nomenclature, in earlier readings. A chemical equation is an expression of a chemical process. For example: AgNO 3(aq) + NaCl(aq) ---> AgCl(s) + NaNO 3(aq) In this equation, AgNO 3 is mixed with NaCl.

The equation shows that the (AgNO 3 and NaCl) react through some process (--->Download game sepak bola winning eleven 2013. ) to form the (AgCl and NaNO 3). Since they undergo a chemical process, they are changed fundamentally. Often are written showing the that each substance is in. The (s) sign means that the compound is a solid. The (l) sign means the substance is a liquid.
The (aq) sign stands for aqueous in water and means the is dissolved in water. Finally, the (g) sign means that the compound is a gas. Coefficients are used in all chemical equations to show the relative amounts of each substance present. This amount can represent either the relative number of, or the relative number of moles (described below). If no coefficient is shown, a one (1) is assumed. On some occasions, a variety of information will be written above or below the arrows. This information, such as a value for temperature, shows what conditions need to be present for a reaction to occur.
For example, in the graphic below, the notation above and below the arrows shows that we need a chemical Fe 2O 3, a temperature of 1000° C, and a pressure of 500 atmospheres for this reaction to occur. The graphic below works to capture most of the concepts described above.
...'>Stoichiometry Program(21.12.2018)Stoichiometry Course Chapters • (REQUIRED FOR CREDIT!) • • • • • • Section Tests Useful Materials Online Calculators Related Information Links Stoichiometry Stoichiometry is simply the math behind chemistry. Given enough information, one can use stoichiometry to calculate masses, moles, and percents within a chemical equation. • • • • • • • • In chemistry, we use symbols to represent the various chemicals. Success in chemistry depends upon developing a strong familiarity with these basic symbols.
Stoichiometry measures these quantitative relationships, and is used to determine the amount of products and reactants that are produced or needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction.
For example, the symbol \'C'represents an atom of carbon, and \'H' represents an atom of hydrogen. To represent a molecule of table salt, sodium chloride, we would use the notation \'NaCl\', where \'Na\' represents sodium and \'Cl\' represents chlorine. We call chlorine \'chloride\' in this case because of its connection to sodium. You should have reviewed naming schemes, or nomenclature, in earlier readings. A chemical equation is an expression of a chemical process. For example: AgNO 3(aq) + NaCl(aq) ---> AgCl(s) + NaNO 3(aq) In this equation, AgNO 3 is mixed with NaCl.

The equation shows that the (AgNO 3 and NaCl) react through some process (--->Download game sepak bola winning eleven 2013. ) to form the (AgCl and NaNO 3). Since they undergo a chemical process, they are changed fundamentally. Often are written showing the that each substance is in. The (s) sign means that the compound is a solid. The (l) sign means the substance is a liquid.
The (aq) sign stands for aqueous in water and means the is dissolved in water. Finally, the (g) sign means that the compound is a gas. Coefficients are used in all chemical equations to show the relative amounts of each substance present. This amount can represent either the relative number of, or the relative number of moles (described below). If no coefficient is shown, a one (1) is assumed. On some occasions, a variety of information will be written above or below the arrows. This information, such as a value for temperature, shows what conditions need to be present for a reaction to occur.
For example, in the graphic below, the notation above and below the arrows shows that we need a chemical Fe 2O 3, a temperature of 1000° C, and a pressure of 500 atmospheres for this reaction to occur. The graphic below works to capture most of the concepts described above.
...'>Stoichiometry Program(21.12.2018)